Introduction
Barraquer has always been a pioneer in the research of new surgical techniques and in the development of new drugs that help restore the eyesight and cure multiple eye conditions.
Clinical trials are studies devised to research new medication or techniques. They aim to bring improvements to the treatment of diseases or treat conditions that currently do not have a cure. They offer the possibility of accessing new treatment free of charge for the patient and for up to three years.
Barraquer has always been dedicated to research and innovation in the field of ophthalmology.
In each clinical trial, the patient must meet certain selection criteria. Not all patients are candidates to participate in a clinical trial. The ophthalmologist will evaluate the suitability of each candidate.
All the clinical studies conducted at the Barraquer Centre have been approved by a Drug Research Ethics Committee (CEIm), a multidisciplinary body independent from the Centre. Clinical trials are conducted based on strict ethical and scientific principles and in compliance with national and international regulations that protect the rights, safety and well-being of the individuals who participate in them.
How do I take part in a clinical trial?
To take part in a trial, you should follow these steps:
- Fill out the application form at the bottom of this page.
- The clinical trial coordinator will contact you for more information and to arrange an appointment with one of our ophthalmologists.
- Once you have completed your appointment with the ophthalmologist and if you meet the criteria to participate in the clinical trial, the project will be explained to you and you will be provided with the necessary information.
- If you decide to participate, you should contact the person in charge of the clinical trials area, who will coordinate the first visit to start your participation in the study.
Duration and cost
Each clinical trial has its own specific duration outlined by the protocol. Some may last a few days or weeks while others may be up to three years long.
The patients selected for the clinical trial will receive all necessary treatment, visits, and follow-up tests COMPLETELY FREE OF CHARGE. In certain studies, the promoter will cover the transport costs of the patients, as long as they reside in the Spanish peninsula.
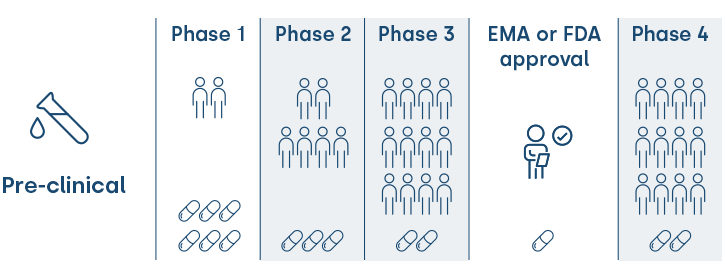
Phases of a clinical trial
The development phases of a clinical trial are:
- Phase 1 > The safety of a drug is assessed on a small number of healthy individuals without the pathology.
- Phase 2 > The drug is studied on a high number of individuals affected by the condition for an initial analysis of its safety and efficacy.
- Phase 3 > The effects of the drug are studied on a higher number of patients –which may include thousands– with the pathology aiming to be treated. It tests the safety and efficacy of the drug before its commercialisation.
- Phase 4 or post marketing surveillance trials > The drug has been approved for sale. Long-term studies are conducted on the safety and efficacy of individuals affected by the condition. This allows for any low incidences of adverse reactions to be detected.
Clinical trials at phases 2, 3 and 4 are currently being conducted the Barraquer Ophthalmology Centre.
