Barraquer supports a culture of research among young people
03/04/2025
27/04/2023
Retrospective studies are investigations that evaluate situations of routine clinical practice with the aim of improving the clinical management of patients. In medicine, we must always ask ourselves if the treatment of patients can be improved, even if it has been previously analysed and protocolised. Testing hypotheses about associations between a clinical event and exposure to a treatment or patient condition will help determine the best therapeutic option in each case.
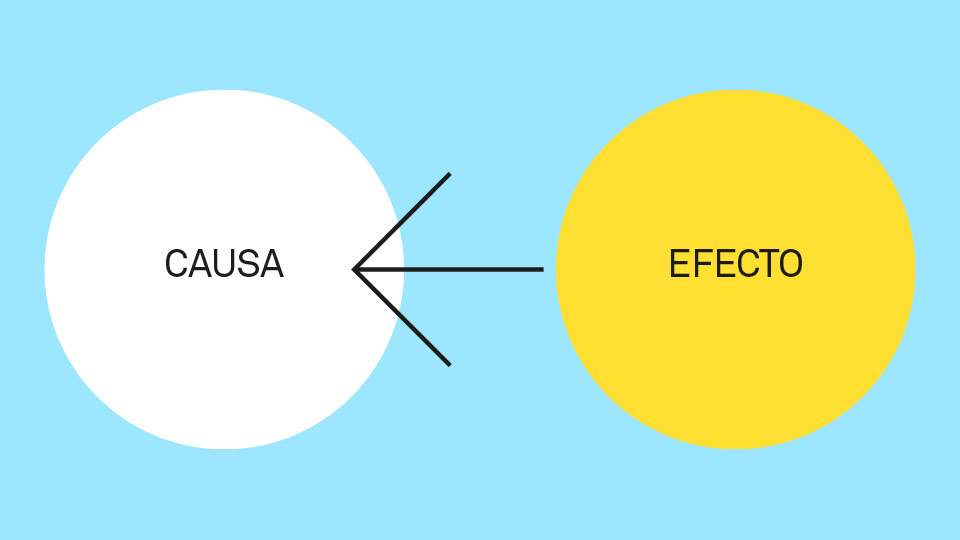
Methodologically speaking, retrospective studies establish the first evidence of a cause-effect relationship, comparing cases and controls. An example of this type of study, in the field of ophthalmology, could be the identification of adverse reactions of low or very low incidence with the use of intravitreal injections, to treat age-related macular disease. These types of events are difficult to detect in pre-marketing clinical trials, since the number of patients is relatively small, for ethical reasons.
The quality of retrospective studies depends on: a clear research question; a controlled selection of the sample (considering all the relevant characteristics to obtain reliable results); consistent and accurate data collection (comprehensive chart review); an adequate analysis and, finally, a clear and precise communication of the results to the scientific community.
At the Barraquer Ophthalmology Centre, we pay special attention to the quality of the retrospective studies we carry out. We work rigorously, determining possible limitations. It is a job that requires specific dedication and collaboration between ophthalmologists and the Research Department.
Eva Calpe, Clinical Research Coordinator at the Barraquer Ophthalmology Centre